Receive valuable peer feedback and grow your research into an endorsed project.




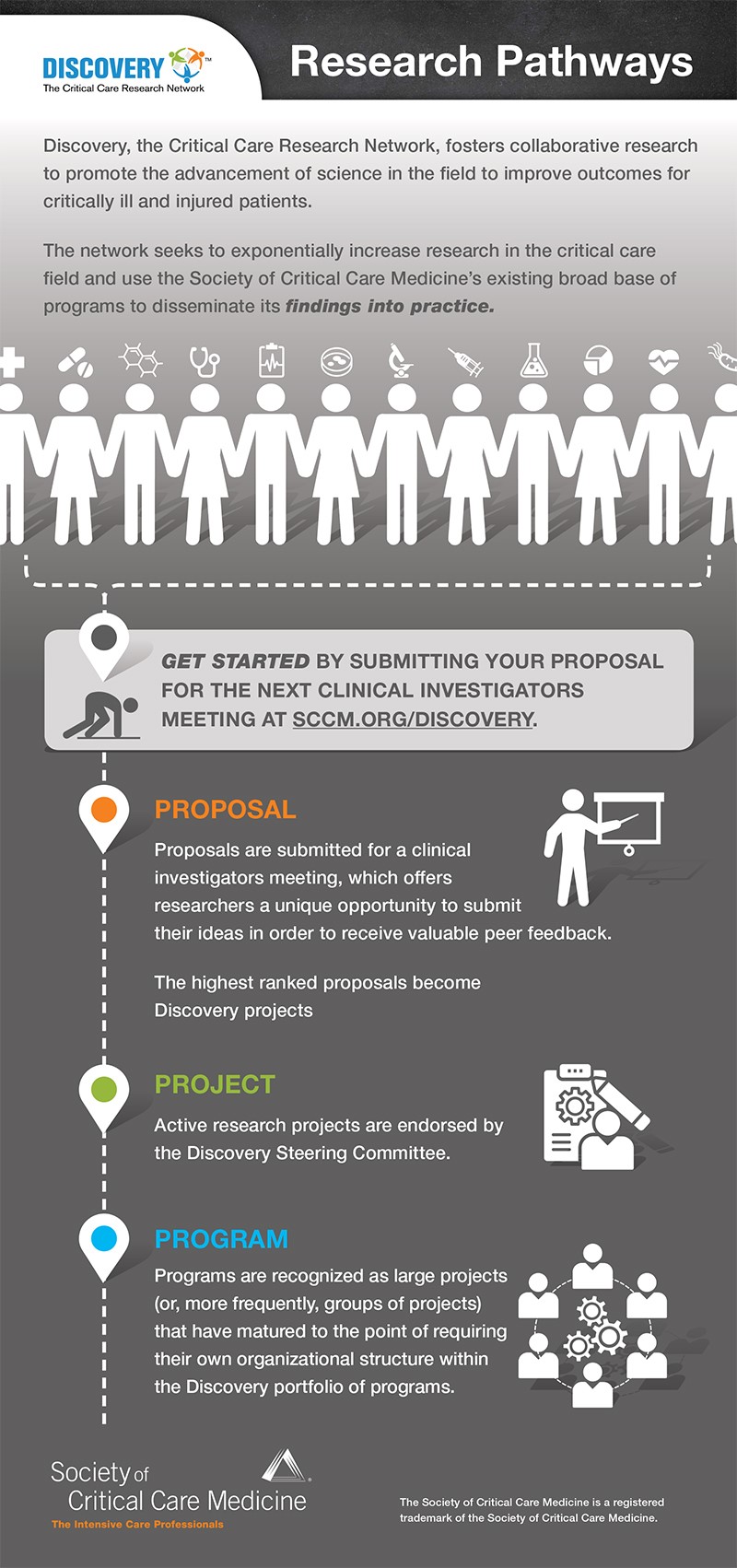 Showcase your research by submitting a Clinical Investigator Proposal to Discovery! Submitting your proposal offers the opportunity to present your proposal during a Clinical Investigators Meeting to gain valuable peer feedback and have your proposal become an Endorsed Project or a Discovery Program.
Showcase your research by submitting a Clinical Investigator Proposal to Discovery! Submitting your proposal offers the opportunity to present your proposal during a Clinical Investigators Meeting to gain valuable peer feedback and have your proposal become an Endorsed Project or a Discovery Program.
Preview the submission form. (Note: All proposals must be submitted via the link above. Proposals will not be accepted by email or U.S. mail.) If you have questions, please contact discovery@sccm.org.
Proposals are reviewed and evaluated by members of the Discovery Steering Committee. In addition to an overall impact score, investigators are provided with a score and comments in the areas of significance, investigator, innovation, approach, and environment.
Discover new and upcoming research, hear from program directors of funding agencies, and learn from other critical care researchers.
Find a list of current Endorsed Projects, Discovery Programs, and published works. Learn about the benefits of submitting a proposal to Discovery and having your proposal become an Endorsed Project.
Proposals not ranked highly after the Discovery Steering Committee’s review will not be provided a letter of support.
Applicants are encouraged to continue to work closely with the Discovery Steering Committee and join the SCCM Discovery community to continue to improve their proposals or execute their projects.